This post is about the end of 2017 and the beginning of more hope. In a moment, I’ll get to the reasons for more hope, but first, I want to describe my version of hope. It’s not something I take on quickly. It’s a slower and “skeptical hope,” which needs clarification and possibly some rambling.
The word “skeptical” is often used when the word “cynical” is more accurate. Being “skeptical” means not easily convinced. It also means asking for carefully collected evidence that stands up to scrutiny, and being skeptical respects the danger in unfounded opinions—especially passionate and unsupported opinions. In popular culture, the word “skeptical” is often used as a poor replacement for the word “cynical.” Calling someone a cynic seems to be like calling someone a liar, which news sources avoid. I’m happy to be a skeptic, and I’m rarely a cynic. Skeptical means that careful analysis shows if an idea is good or bad. Cynical means all or most ideas are bad.
With cancer treatments, my skeptical hope comes from doctors who have seen treatments work and fail with thousands of people and during decades of time. These doctors have learned the value of being skeptical. Many of them carefully study medical successes and failures, write the details in an article, and other doctors read and critique the article. That’s the process of peer-review. As long as corporations don’t mess with peer-review, especially drug companies, that kind of review gives medicine the best treatments possible, which has extended our lives for decades.
I’ll finally stop rambling, but I think we all need to be more skeptical with medicine, and maybe the rest of life. Finally, I’ll get to the reasons for more skeptical hope.
Following custom, many organizations are sending out their “Best of 2017” news stories. I just received one of those stories from the Prostate Cancer Foundation (PCF). I’m including the first three in this blog post, starting with my own comments before each.
#1: New Life Extending Treatment Protocol
My comments on #1: After making careful plans for a few treatments, I’m now taking abiraterone. Based on this first item, I feel lucky. ADT is mentioned below. It means turning off a guy’s testosterone.
In 2017, results were reported from two separate clinical trials, LATITUDE and STAMPEDE, which tested giving men abiraterone earlier, in combination with ADT, at a time when men would normally just be starting ADT. The results were pretty remarkable: this combination of drugs reduced risk of death by at least 35% and delayed cancer progression by an average of 18 months.
But for some men, it worked even better. “This is practice-changing,” says Jonathan Simons, M.D., CEO of the Prostate Cancer Foundation. “I have never seen a treatment where you could, five years later, see no progression in some men. There are some extreme responders who get a very significant remission. It may be that abiraterone does not just stop cancer from proliferating, but it also stops, or significantly delays, cancer from mutating and becoming more resistant to treatment.”
What this means for patients: This is one of those game-changers that really brings hope to patients with advanced prostate cancer. These results are new, and it may take a while for the practice to pick up steam among medical oncologists. If you think you are at a stage of treatment where this protocol might be useful to you, don’t wait for your doctor to tell you: ASK.
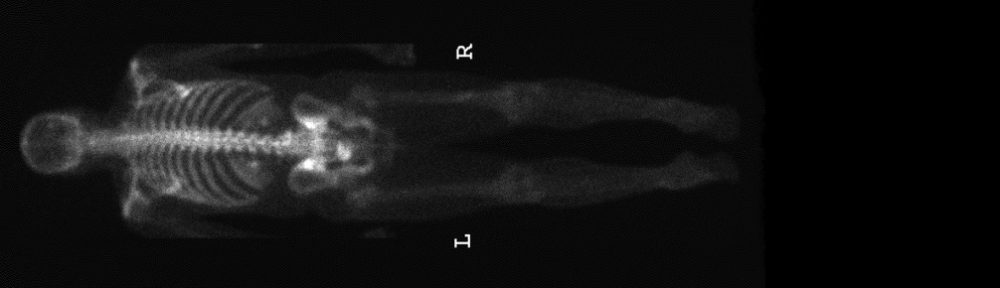
#2: Radioactive Molecules that Hunt and Kill Cancer
My Comments on #2: Like all cancer patients, the hardest news I’ve heard is that my cancer has spread. During those moments, I desperately want a drug that can find the cancer cells and kill them, 2 or 3 times if possible. Even though this treatment sounds exciting, there are some words that are important. It says that “some patients” benefit. Only careful clinical trials can tell which patients benefit and how to get more to benefit.
PSMA, or prostate-specific membrane antigen, is a protein that is primarily found on the surface of prostate and prostate cancer cells. The protein becomes like a unique signature that can be tracked by small molecules or proteins that are injected into your bloodstream. Researchers discovered that if you attach a radioactive isotope to the PSMA-targeting molecule (together known as a “radiopharmaceutical”), you have a particularly effective way of hunting down and killing prostate cancer cells that are outside the prostate.
In other words, instead of putting a pill in your body, doctors inject a radioactive pharmaceutical that acts like a homing device, traveling through the body until it reaches its target: PSMA. These particles are particularly good at accurately identifying – and killing – prostate cancer metastasis throughout the body.
This is huge.
Some patients undergoing PSMA radionuclide therapy have seen tumor metastases shrink to amazing degrees. However, standardized clinical trials have yet to be conducted for these new treatments, to definitively prove efficacy and establish the best way to deliver this treatment.
#3: Faster Clinical Trials
My comments on #3: I don’t want medical trials to be rushed because that leads to deadly risks, but going too slow is also a risk, which is why this one is important.
In order for the FDA to approve a new therapy, an improvement in length or quality of life due to the therapy must first be demonstrated in clinical trials. The “overall survival” (OS) endpoint, which measures the length of time from randomization to death from any cause, is the gold standard for measuring the impact of a treatment on length of life. In localized prostate cancer, reaching an OS endpoint can require 10-15 years — a prohibitive timeframe for pharmaceutical companies. This fact has translated into only limited improvements being made in the treatment of early, aggressive prostate cancer in the last decade.
PCF identified this issue as a critical unmet need, and in 2012, supported the establishment of a working group called ICECaP (Intermediate Clinical Endpoints of Cancer of the Prostate), an international collaborative initiative to undertake the arduous task of identifying an intermediate clinical trial endpoint that can accurately predict overall survival but could be obtained much earlier in the course of the disease.
Establishment of an intermediate “surrogate” endpoint would hasten clinical trials and regulatory approvals for new therapies for early prostate cancer patients.
If you’re interested in the entire list of the 5 best advances in 2017, click here. Happy Holidays everybody! And I say that with all kinds of hope. 🙂
This is great news! Thanks for sharing. Made me smile the entire time I read it. I am so glad that there continues to be so many new advances in treatment. I will keep good thoughts and have “skeptical” hope with you!